2013
Cerebral vasculature, Alzheimer's disease and cerebral amyloid angiopathy: What is the Connection: A Mathematical Model
C. Kyrtsos and J. Baras
Proceedings of 6th International IEEE EMBS Conference on Neural Engineering, San Diego, California, November 6-8, 2013.
Poster
Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60-80% of all dementias. AD is associated with loss of short term memory, changes in mood and sleep patterns, and loss of voluntary muscle control. Histologically, post-mortem brain sections of affected individuals show increased levels of beta amyloid (Aβ) deposited throughout the brain parenchyma and neurofibrillary tangles (NFTs) within neurons [1]. Elevated levels of neuroinflammatory markers and activated microglia are also present.
Another important histological finding in AD is the presence of cerebral amyloid angiopathy (CAA) in nearly 90% of AD patients [1]. This presents as the deposition and build-up of Aβ within brain endothelial cells, as well as at the blood-brain barrier (BBB). The exact role of CAA in AD pathogenesis is not well understood, though it is known to increase the permeability of the BBB, increasing the transport of neurotoxins into the brain and possibly stimulating an inflammatory response or apoptosis. Conversely, if an Aβ plaque deposition within the cerebral vasculature begins to occlude the vessel, ischemia and subsequent neuronal cell loss may result.
In this paper, a mathematical model studying the possible interactions between CAA and AD pathology is developed. Previous mathematical models have studied the effects of Aβ transport rate on the development of CAA and looked at the effects of cerebral blood flow on AD pathology [2-4], however, no models have directly studied how CAA may be a contributory process in AD pathogenesis. The blood-brain barrier has been modeled as a single layer of endothelial cells. Transvascular transport of fluid across the BBB was defined by the Starling equation:
![]()
where L is the lumen, I is the interstitial space, p is the hydrostatic pressure, π is the oncotic pressure, σ is the osmotic reflection coefficient, and S/V is the surface area to volume ratio. Michaelis-Menten kinetics were used to study the binding of glucose to the GLUT receptor:
![]()
where Vmax is the maximum velocity of solute binding, [S] is the concentration of glucose and Km is the Michaelis constant. The model developed here specifically studies how deposition of Aβ in cerebral vasculature affects BBB 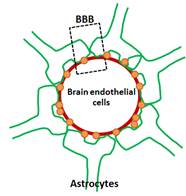 permeability (with subsequent neuronal cell death) and how local neuronal cell density can change as a function of the extent of Aβ deposition. Figure 1 (below) demonstrates the scenarios studied by this model. Future models will study how vessel properties and the immune cells play a role in this process.
permeability (with subsequent neuronal cell death) and how local neuronal cell density can change as a function of the extent of Aβ deposition. Figure 1 (below) demonstrates the scenarios studied by this model. Future models will study how vessel properties and the immune cells play a role in this process.